
Food Safety
Did You Know About Hygiena's Innovate System for Aseptic and Finished Product Testing?
Hygiena is not only engaged in PCR (Polymerase Chain Reaction) and Environmental Monitoring methods to help improve food safety across the industry, but Hygiena also supports another ATP-based method in the Innovate System for aseptic and finished dairy and beverage product manufacturers. State of the Food & Beverage Packaging Industry The global aseptic food and beverage packaging market is expected to reach $35 billion by 2025. Some of this growth is due to the increased popularity in dairy products. This has fueled the demand for products with longer shelf lives and room temperature storage. As a result, the demand for aseptically packaged beverages has grown significantly. Along with the demand for aseptically packaged beverage products, the demand for improved technology to support sustainability and efficiency initiatives has grown as well.
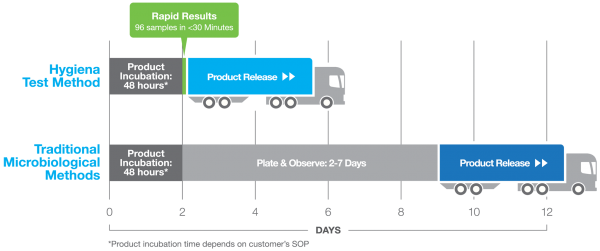
Historically, finished aseptic dairy and beverage products have been tested utilizing traditional agar and pH methods, but within the past 20 years rapid methods have made an appearance, and are widely adopted for the detection and release of finished aseptic dairy and beverage products. When we look at the reasons why, concerns with data integrity, time to result, and overall operational costs are key indicators of this trend. Cost-Savings Generated with Rapid Method When we spoke to large dairy producers in Europe, they mentioned by simply implementing the Innovate rapid method to replace their traditional agar-based method, and they were able to generate a cost savings of ~$450k after five years, with a return on investment of only 6-9 months! The shorter time to result delivered actionable information much faster than traditional methods, allowing them to confirm product quality sooner, and therefore ship to market faster in a process that shaved days off their inventory and production cycle times. While this customer’s main driver was the faster turnaround time and recognition of revenue, another driver of the Innovate implementation was the data integrity aspect of the technology. When manufacturing companies are going through the testing process of their finished product, the results must be recorded in an impartial, unbiased way. Traditionally, as was the case with this customer, the results were recorded manually, and the lab technician had to manually upload these results into an excel sheet. I have been to some customer sites where they hired a single FTE for this data input based on the number of data results tested in a single day. The concern with this method is it is quite easy to make a user error when inputting the data. The data is not controlled or protected, making this method prone to user error. With the Innovate System, all results are automatically encrypted and stored, either locally or directly to the customer’s LIMS, and cannot be edited - taking away any risk of user error or data corruption.
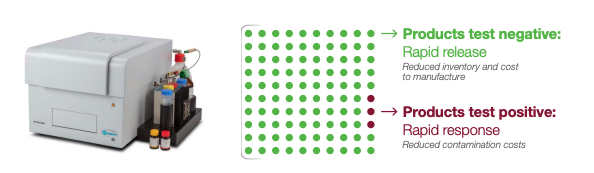
Another driver behind the transition to rapid methods was the improved sustainability impact rapid methods bring to the table compared with traditional agar plates. The energy and resources required to carry out traditional agar plating methods are far greater than utilizing rapid method diagnostics. By transitioning to the Innovate System, this customer was able to save on energy costs through the reduction of autoclave use and incubator space required to run finished product testing, in addition to a significant reduction of waste materials. When it comes to confirming the quality of your product, consider the Innovate System for its operational efficiencies, ease of use, and access to industry-leading technical and scientific support. Not to mention the significant cost savings potential! To learn more, visit our website at hygiena.com/instruments-and-automation/monitoring-systems/innovate.