
Food Safety
4 Reasons to Choose Pathogen Quantification Over Detection
Key Takeaways:
- Pathogen Quantification can provide a full story of the situation, additional value to the industry and academia
- Quantification applications can help determine the source of contamination within processing facilities and allow for swift decisions
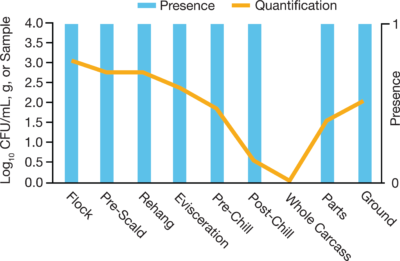
In the meat and poultry industry, it is essential to have a robust Salmonella testing program along with other potentially harmful pathogens. For years, these food processors have tested the same way, using PCR or standard plating methods to identify contamination throughout their processing operations. However, prevalence (presence/absence) results do not indicate how many organisms are present in any positive samples - it could have been a low count of a few cells or a high count of many cells per positive sample. Recently, the door has opened for not only pathogen detection but also quantification to understand the level of contamination present.
-
Pathogen Quantification Provides A Complete Story
- Quantification defines what is happening at each processing step at each facility, allowing for more targeted interventions to reduce pathogen counts. Facilities can focus on addressing specific areas while allowing controlled production processes to continue.
- Quantification also warrants the setting of baseline standards to ensure food is safe for consumption. When paired with prevalence testing, the collective data can identify specific areas for improvement. It could be as simple as pinpointing a single step in the process where cross-contamination is occurring. On the opposite end of the spectrum, high prevalence rates but low pathogen numbers may mean process control may be failing or that more antimicrobial cleaning is needed to reduce loads.
- The quantification data defines the situation in detail so proper action can be taken to remove the source(s) of contamination and possibly prevent future occurrences.
- By quantifying organism numbers, the processing facility can determine if interventions are working to reduce pathogen loads or if new processes need to be developed to reduce prevalence.
-
Pathogen Quantification Brings Value to Academia and Industry
- Quantification will remain at the forefront as academia and industry continue to generate data demonstrating its value to the meat and poultry industries. On October 19, 2021, the USDA’s Food Safety and Inspection Service (FSIS) announced that it intends to initiate several key activities to help reduce Salmonella This involves seeking stakeholder feedback on specific Salmonella control and measurement strategies, including how to reduce Salmonella contamination coming into the facility.
- The National Advisory Committee for Microbiological Criteria in Foods, an independent federal advisory committee, will be advising FSIS on how they can use the latest scientific research to improve its approach to Salmonella The agency stated, “Since it is not just the presence or absence of Salmonella, but the quantity of bacteria that can impact the likelihood of illness, FSIS will examine how quantification can be incorporated into this approach.”

-
Pathogen Quantification Can Determine the Source of Contamination Within Processing
- Aligned with this new USDA/FSIS approach, the first rapid pathogen enumeration method (SalQuant™) recently received AOAC-RIPTM
- SalQuant™ utilizes the BAX ® System Real-Time PCR Assay for Salmonella to quantitate pathogen numbers.
- Protocols are now available for over 14 poultry matrices, allowing the evaluation of pathogen load at each step in processing; more protocols are coming soon for pork and beef matrices.
- To validate the importance of this technology, IAFP recently presented Hygiena™ with the 2021 IAFP Innovation Award for the development of the SalQuant™ enumeration method.
- Using a single system, testing can be done for both prevalence (limits) and quantitation (load). The BAX ® System Real-Time PCR Assay for Salmonella was previously shown to be equivalent to the reference methods for Salmonella detection, with thresholds of 10 CFU/g (LOD10) for all product types.
- The SalQuant™ method has been validated for Salmonella quantification in multiple matrices, including ground poultry, poultry carcass rinsates and caeca, ground beef and beef trim, and ground pork and pork trim. With SalQuant™, the lower limit of enumeration is 1 CFU/g (LOD1) for most sample types with an incubation range of 3-12 hours at 42˚C, depending on the product type being tested.

- Pathogen Quantification Allows Plant Managers to Act Quickly
- This method allows plant managers to accurately detect contamination and quantify levels in meat and poultry products rapidly, easily, and effectively.
- By quantifying results instead of only detecting presence, processors will not only know if a product lot is positive for Salmonella, but they can also determine how much Salmonella is present. This information allows them to make informed, critical decisions about whether to further process the product for sale.
What can quantification do for you? By tracking pathogen numbers rather than just presence/absence, personnel can fully understand what is happening in the processing facility, from entry to final packaging. Operations managers can now pinpoint the source of increased pathogen levels and proactively take measures to ensure the contamination is eliminated at the source and not spread throughout the facility. Numbers will indicate if the contamination is already present at the entry into the facility or if it is introduced during the processing within the facility. This means more poultry are safely released for sale to the consumer. Learn how the BAX ® System SalQuant™ solution can solve challenging pathogen issues in your facility.